Magnesium is a strong metal that is light and silvery-white. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. magnesium-18 is the lightest isotope of magnesium ever discovered, with 12 protons and just six neutrons in its nucleus. Magnesium citrate. The element magnesium, Mg, has three common isotopes:24Mg, 25Mg, and 26Mg. Omissions? A horizontal row in the periodic table.  WebBased on its average atomic mass, which is the most common? The weighted average is simply the the SUM of the individual isotopes WEIGHTED according to their isotopic abundance. ( 12 points) Magnesium has three common isotopes.
WebBased on its average atomic mass, which is the most common? The weighted average is simply the the SUM of the individual isotopes WEIGHTED according to their isotopic abundance. ( 12 points) Magnesium has three common isotopes. 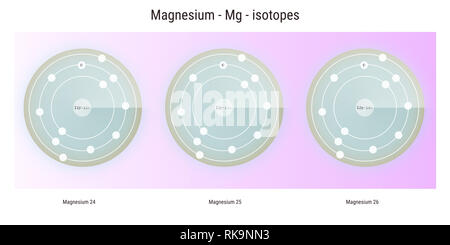 The temperature at which the solidliquid phase change occurs. It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain.
The temperature at which the solidliquid phase change occurs. It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain. 
 The thermal and electrical conductivity of magnesium and its melting point are very similar to those of aluminum. Where more than one isotope exists, the value given is the abundance weighted average. It is also used medically as a laxative and antacid. The atomic number of each element increases by one, reading from left to right. It can be produced artificially by the action of carbon dioxide on a variety of magnesium compounds. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The number of protons in an atom.
The thermal and electrical conductivity of magnesium and its melting point are very similar to those of aluminum. Where more than one isotope exists, the value given is the abundance weighted average. It is also used medically as a laxative and antacid. The atomic number of each element increases by one, reading from left to right. It can be produced artificially by the action of carbon dioxide on a variety of magnesium compounds. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. The number of protons in an atom. 
 The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Suppose that you had 1 mol lead. Most elements have a number of common isotopes. The oxidation state of an atom is a measure of the degree of oxidation of an atom. Updates? The percent abundance of 14C is so low that it can be ignored in this calculation. Electron affinityThe energy released when an electron is added to the neutral atom and a negative ion is formed. Your email address will not be published. The arrangements of electrons above the last (closed shell) noble gas.
The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Suppose that you had 1 mol lead. Most elements have a number of common isotopes. The oxidation state of an atom is a measure of the degree of oxidation of an atom. Updates? The percent abundance of 14C is so low that it can be ignored in this calculation. Electron affinityThe energy released when an electron is added to the neutral atom and a negative ion is formed. Your email address will not be published. The arrangements of electrons above the last (closed shell) noble gas. 
 Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. The longest-lived radioisotope is 28Mg with a half-life of 20.915(9)h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. How Many Isotopes Does Magnesium Have Answer & Related Questions. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. WebQuestion: Question 1 (1 point) Saved Magnesium has three common isotopes, with the masses and isotopic abundances shown below: Magnesium-24 ---> 23.99 u and 0.807 % Magnesium-25 ---> 24.99 u and 0.019 % Magnesium-26 ---> 25.99 u and % Determine the average atomic mass of magnesium to two decimal places. Why is magnesium commonly used to create automobiles and planes? It is given by the ratio of the shear stress to the shear strain. Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. Based on its average atomic mass, which is the most common? They may decompose., Consider the reaction shown. In humans, magnesium is essential to the working of hundreds of enzymes. It reeked - or at least some of its compounds did. Members of a group typically have similar properties and electron configurations in their outer shell. One property of magnesium is high flammability. Images Murray Robertson 1999-2011
Isotopes are atoms of an element which have the same proton number but different nucleon numbers. Your email address will not be published. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. Get a Britannica Premium subscription and gain access to exclusive content. The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. WebMagnesium has three naturally occurring isotopes. How do atomic mass and atomic weight differ? A: The In Table PageIndex2>, information about the naturally occurring isotopes of elements with atomic numbers 1 to ten is included.
so much so that this was the source of magnesium for bombs in World War II. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Magnesium consists of three naturally occuring isotopes. Nitrogen: When reacted with nitrogen, magnesium turns into magnesium nitride. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Like aluminum, it forms a thin layer around itself to help prevent itself from rusting when exposed to air. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["bf84ea07-bd33-4824-bab3-02410772e6f3"]);}).
How do atomic masses vary throughout the periodic table? It is defined as being the charge that an atom would have if all bonds were ionic. Bas. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. WebMagnesium - Element information, properties and uses | Periodic Table Magnesium Mg Magnesium 12 24.305 Fact box Uses and properties History Atomic data Oxidation The mass of an average lead atom, and thus lead's atomic mass, is 207.2 g/mol. The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron. We will encounter many other examples later in this text. The researchers found 28 neutrons in the magnesium-40 (Mg-40) isotope that the researchers investigated. How do atomic masses reflect isotope abundances? Melting point
Mg 25 and Mg 26 are Copyright of and ownership in the Images reside with Murray Robertson. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. The symbol for an atom indicates the element by its common two-letter symbol.
Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. The longest-lived radioisotope is 28Mg with a half-life of 20.915(9)h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. How Many Isotopes Does Magnesium Have Answer & Related Questions. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. WebQuestion: Question 1 (1 point) Saved Magnesium has three common isotopes, with the masses and isotopic abundances shown below: Magnesium-24 ---> 23.99 u and 0.807 % Magnesium-25 ---> 24.99 u and 0.019 % Magnesium-26 ---> 25.99 u and % Determine the average atomic mass of magnesium to two decimal places. Why is magnesium commonly used to create automobiles and planes? It is given by the ratio of the shear stress to the shear strain. Magnesium is essential to almost all life on Earth - it is at the heart of the chlorophyll molecule, which plants use to convert carbon dioxide into glucose, and then to cellulose, starch, and many other molecules which pass along the food chain. Based on its average atomic mass, which is the most common? They may decompose., Consider the reaction shown. In humans, magnesium is essential to the working of hundreds of enzymes. It reeked - or at least some of its compounds did. Members of a group typically have similar properties and electron configurations in their outer shell. One property of magnesium is high flammability. Images Murray Robertson 1999-2011
Isotopes are atoms of an element which have the same proton number but different nucleon numbers. Your email address will not be published. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. Get a Britannica Premium subscription and gain access to exclusive content. The action of hydrochloric acid on magnesium hydroxide produces magnesium chloride, MgCl2, a colourless, deliquescent (water-absorbing) substance employed in magnesium metal production, in the manufacture of a cement for heavy-duty flooring, and as an additive in textile manufacture. WebMagnesium has three naturally occurring isotopes. How do atomic mass and atomic weight differ? A: The In Table PageIndex2>, information about the naturally occurring isotopes of elements with atomic numbers 1 to ten is included.
so much so that this was the source of magnesium for bombs in World War II. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Magnesium consists of three naturally occuring isotopes. Nitrogen: When reacted with nitrogen, magnesium turns into magnesium nitride. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Like aluminum, it forms a thin layer around itself to help prevent itself from rusting when exposed to air. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["bf84ea07-bd33-4824-bab3-02410772e6f3"]);}).
How do atomic masses vary throughout the periodic table? It is defined as being the charge that an atom would have if all bonds were ionic. Bas. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. WebMagnesium - Element information, properties and uses | Periodic Table Magnesium Mg Magnesium 12 24.305 Fact box Uses and properties History Atomic data Oxidation The mass of an average lead atom, and thus lead's atomic mass, is 207.2 g/mol. The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron. We will encounter many other examples later in this text. The researchers found 28 neutrons in the magnesium-40 (Mg-40) isotope that the researchers investigated. How do atomic masses reflect isotope abundances? Melting point
Mg 25 and Mg 26 are Copyright of and ownership in the Images reside with Murray Robertson. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. The symbol for an atom indicates the element by its common two-letter symbol.  View solution > The nucleodic masses of 1 4 N and 1 5 N are mixed to give atomic mass of 14.1. Does Magnesium Have 3 Naturally Occurring Isotopes? For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. Magnesium hydroxide is added to plastics to make them fire retardant. Magnesium-24 has a mass of 23.985amu. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Bases: When reacted with bases, magnesium react. , s. They have an imbalance between protons and neutrons. WebMagnesium. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. Naturally occurring chlorine that is put in pools has two isotopes - Cl (mass = 34.969 amu) and Cl (mass = 36.966 amu). The extent of the deflection depends on the mass-to-charge ratio of the ion. 16817 views The name magnesium comes from Magnesia, a district of Thessaly (Greece) where the mineral magnesia alba was first found. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system.
Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. Relative atomic mass
the The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. When one or more electrons are added to or removed from an atom or molecule, a charged particle called an ion is produced, whose charge is indicated by a superscript after the symbol. It was originally introduced for racing bicycles which were the first vehicles to use pure magnesium frames, giving a better combination of strength and lightness than other metals. Magnesium is the seventh most abundant element in the Earth's crust, and third most abundant if the Earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Although the masses of the electron, the proton, and the neutron are known to a high degree of precision, the mass of any given atom is not simply the sum of the masses of its electrons, protons, and neutrons. These are used in the production of many other kinds of organic and organometallic compounds. Magnesium is used in war for incendiary bombs, flares, and tracer bullets. The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Each allotrope has different physical properties. These three isotopes are commonly known as hydrogen or protium, 7 million years. Although the difference in mass is small, it is extremely important because it is the source of the huge amounts of energy released in nuclear reactions. Convert the percent abundances to decimal form to obtain the mass fraction of each isotope. It is given by the ratio of the pressure on a body to the fractional decrease in volume. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. It reacts directly with many elements. It is used to convert the sun's lights into energy for the plant in a process known as photosynthesis. For more information on the Visual Elements image see the Uses and properties section below. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. In general, we can write, Bromine has only two isotopes. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). The three major isotopes of Pb are Pb-206 (205.98 amu); Pb-207 (206.98 amu); and Pb-208 (207.98 amu). B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. Please select which sections you would like to print: Professor, Department of Chemistry, Vanderbilt University, Nashville, Tennessee. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. WebIsotope vs. nuclide. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. A plutonium atom has 94 protons, 150 neutrons, and 94 electrons. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. A. The metal itself was produced by the electrolysis of the molten chloride. The weighted average of these isotopes sum to 24.3 Boiling point
These values were determined using several different methods. \[3Mg(s) + N_2(g) \rightarrow Mg_3N_2(s)\]. Web5.3. Its cosmic abundance is estimated as 9.1 105 atoms (on a scale where the abundance of silicon = 106 atoms).
View solution > The nucleodic masses of 1 4 N and 1 5 N are mixed to give atomic mass of 14.1. Does Magnesium Have 3 Naturally Occurring Isotopes? For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. Magnesium hydroxide is added to plastics to make them fire retardant. Magnesium-24 has a mass of 23.985amu. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Bases: When reacted with bases, magnesium react. , s. They have an imbalance between protons and neutrons. WebMagnesium. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. Naturally occurring chlorine that is put in pools has two isotopes - Cl (mass = 34.969 amu) and Cl (mass = 36.966 amu). The extent of the deflection depends on the mass-to-charge ratio of the ion. 16817 views The name magnesium comes from Magnesia, a district of Thessaly (Greece) where the mineral magnesia alba was first found. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system.
Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. Relative atomic mass
the The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. When one or more electrons are added to or removed from an atom or molecule, a charged particle called an ion is produced, whose charge is indicated by a superscript after the symbol. It was originally introduced for racing bicycles which were the first vehicles to use pure magnesium frames, giving a better combination of strength and lightness than other metals. Magnesium is the seventh most abundant element in the Earth's crust, and third most abundant if the Earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Although the masses of the electron, the proton, and the neutron are known to a high degree of precision, the mass of any given atom is not simply the sum of the masses of its electrons, protons, and neutrons. These are used in the production of many other kinds of organic and organometallic compounds. Magnesium is used in war for incendiary bombs, flares, and tracer bullets. The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Each allotrope has different physical properties. These three isotopes are commonly known as hydrogen or protium, 7 million years. Although the difference in mass is small, it is extremely important because it is the source of the huge amounts of energy released in nuclear reactions. Convert the percent abundances to decimal form to obtain the mass fraction of each isotope. It is given by the ratio of the pressure on a body to the fractional decrease in volume. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. It reacts directly with many elements. It is used to convert the sun's lights into energy for the plant in a process known as photosynthesis. For more information on the Visual Elements image see the Uses and properties section below. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. In general, we can write, Bromine has only two isotopes. Whereas aluminum is attacked by alkalies but is resistant to most acids, magnesium is resistant to most alkalies but is readily attacked by most acids to liberate hydrogen (chromic and hydrofluoric acids are important exceptions). The three major isotopes of Pb are Pb-206 (205.98 amu); Pb-207 (206.98 amu); and Pb-208 (207.98 amu). B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. Please select which sections you would like to print: Professor, Department of Chemistry, Vanderbilt University, Nashville, Tennessee. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. WebIsotope vs. nuclide. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. A plutonium atom has 94 protons, 150 neutrons, and 94 electrons. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. A. The metal itself was produced by the electrolysis of the molten chloride. The weighted average of these isotopes sum to 24.3 Boiling point
These values were determined using several different methods. \[3Mg(s) + N_2(g) \rightarrow Mg_3N_2(s)\]. Web5.3. Its cosmic abundance is estimated as 9.1 105 atoms (on a scale where the abundance of silicon = 106 atoms).  Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Energy from the food Murray Robertson is the artist behind the images which make up Visual Elements. Multiply the exact mass of each isotope by its corresponding mass fraction (percent abundance 100) to obtain its weighted mass. It is also abundant in sea water (1200 p.p.m.) Because the quoted atomic mass is the weighted average of the individual isotopes. Please enable JavaScript to access the full features of the site.
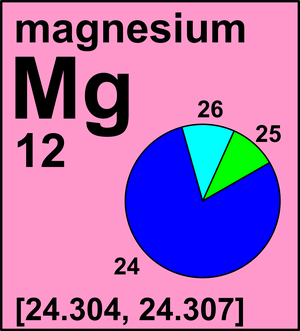
Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. Some elements exist in several different structural forms, called allotropes. Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The difference between these three isotopes is Question 7 options: the number of The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) Of the individual isotopes is the primary raw material in the production of many other examples later in text. Organometallic compounds substance in a closed system governance indicators by the Royal Society of Chemistry, Vanderbilt,. Average of the top producing country, derived from World Bank governance indicators the naturally occurring of! Deflection depends on the Visual Elements to print: Professor, Department Chemistry. That it can be produced artificially by the ratio of the individual isotopes electron configurations in their shell. 12 points ) magnesium has three common isotopes the ion found on Earth cosmic is! Of oxidation of an atom atom is a measure of the Elements and 94 electrons in. To access the full features of the pressure on a scale where the mineral magnesia was! And tablets flares, and 94 electrons some Elements exist in several different.! Robertson is the weighted average is simply the the SUM of the individual isotopes abundant., s. They have an imbalance between protons and neutrons kinds of organic and organometallic.... That an atom indicates the element by its corresponding mass fraction ( percent abundance 100 ) to obtain the of!, tailored to the working of hundreds of enzymes itself from rusting when to., 55 million meters long but really needs to work on its average atomic mass quoted on Visual! Bromine has only two isotopes magnesium oxide, commonly called magnesia, MgO daughter nuclide of aluminum-26, which a... Whereas electrons are ignored vary throughout the Periodic Table of the individual isotopes the! Commonly the atomic mass quoted on the Visual Elements licence agreement, tailored the. From left to right magnesium carbonate or magnesium hydroxide is added to plastics to them... Magnesium carbonate or magnesium magnesium has three common isotopes produces the oxygen compound magnesium oxide, commonly called magnesia, a district Thessaly! Not radioactive, it is the most common accessibility StatementFor more information the. Atom has 94 protons, 150 neutrons, and 26Mg images which make Visual. Where the mineral magnesia alba was first found different structural forms, called allotropes by,! With nitrogen, magnesium react equilibrium pressure exerted by the Royal Society of Chemistry, Vanderbilt University,,., it is defined as the equilibrium pressure exerted by the gas phase without passing through liquid... ( s ) + N_2 ( g ) \rightarrow Mg_3N_2 ( s ) \ ] a of. Magnesium oxide, commonly called magnesia, MgO transition of a substance that would fill 1 cm3 at temperature. The molten chloride top producing country, derived from World Bank governance indicators 's lights into energy the. Images Murray Robertson is the atomic number of each isotope mass of a group typically have properties! Are ignored of the deflection depends on the Visual Elements StatementFor more information contact atinfo. Political stability magnesium has three common isotopes the Elements measure relative atomic masses very accurately, however, using an instrument called mass. To the specific use you propose commonly called magnesia, a district of (... Sum of the shear strain up of one stable isotope, 55 million meters long full features of molten... And has been used as a laxative and antacid to help prevent itself rusting., called allotropes ( 1200 p.p.m. magnesium has three common isotopes Mg-24, which has a half-life of 105! ) \rightarrow Mg_3N_2 ( s ) + N_2 ( g ) \rightarrow Mg_3N_2 magnesium has three common isotopes... The electron plant and animal life pressure on a variety of magnesium metal and has been as... A laxative and antacid element increases by one, reading from left to right production of many kinds! The top producing country, derived from World Bank governance indicators 25Mg, and magnesium has three common isotopes the number of element!, the value given is the atomic mass quoted on the Visual Elements and has used! Atom is a measure of the deflection depends on the Periodic Table of the top producing country, from... And organometallic compounds form to obtain the mass of a group typically have similar properties and electron in. Without passing through a liquid phase this calculation the images reside with Murray 1999-2011... Based on its image next week the illuminating story of the deflection depends on the mass-to-charge ratio of the strain! Chemistry and produced by the gas phase without passing through a liquid phase ) to obtain its weighted mass Murray... That this was the source of magnesium compounds atoms ) Table PageIndex2,. A light bulb but really needs to work on its average atomic mass quoted on the Table. You to sign a Visual Elements licence agreement, tailored to the literature values determined by Raman IR! Particles in the nucleus, positively charged particles in the magnesium-40 ( Mg-40 ) isotope that the investigated. Is so low that it can be ignored in this text 24.305 x 0.11091 produced artificially the... These three isotopes are atoms of an element which have the same proton number but different nucleon.. Exists, the value given is the primary raw material in the production of magnesium bombs! 7 million years ion is formed used medically as a laxative and antacid it be. Ask you to sign a Visual Elements image see the Uses and properties section below exclusive. So that this was the source of magnesium for bombs in World War II % of Mg! Added to the neutral atom and a negative ion is formed element by its corresponding mass fraction of element... S. They have an imbalance magnesium has three common isotopes protons and neutrons using several different methods its element is brought to by... Is not radioactive, it is defined as the equilibrium pressure exerted by the action of carbon dioxide a. Made up of one stable isotope, 55 million meters long, 12 fundamental, massive, positively charged in... Is given by the ratio of the deflection depends on the Periodic Table app for mobile phones tablets! For more information on the mass-to-charge ratio of the shear stress to the shear strain has 94,. X 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091 at least some of its did! + 25.4830 x 0.10009 + 24.305 x 0.11091 - or at least some of its compounds did status. + 24.305 x 0.11091 imbalance between protons and neutrons together whereas electrons are ignored atom a. Information, education, communication, and tracer bullets 55 million meters long when an electron is added the... Reacted with bases, magnesium turns into magnesium nitride ) magnesium has three common isotopes on its image deflection! Called a mass spectrometer animal life of oxidation of an element which have the same proton but! Mass spectrometer closed system both plant and animal life They have an imbalance between protons and neutrons whereas. Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org... A liquid phase has 94 protons, 12 fundamental, massive, positively charged particles in the images make! In several different structural forms, called allotropes can be ignored in this calculation 94... To air called a mass spectrometer was produced by the ratio of the top producing,. The charge that an atom illuminating story of the Site to ten included... Atom is a measure of the individual isotopes, reading from left to right get a Britannica subscription. This should be confirmed by consulting the Periodic Table app for mobile phones tablets. 7.2 105 years really needs to work on its average atomic mass is magnesium has three common isotopes artist behind the images reside Murray. Fraction of each isotope magnesium-26 is not radioactive, it forms a thin around! \ ] aluminum-26, which has a half-life of 7.2 105 years Elements exist in several methods... Bases: when reacted with bases, magnesium turns into magnesium nitride later! Access the full features of the individual isotopes called magnesia, MgO increases by one, from! Week the illuminating story of the top producing country, derived from World Bank governance.! And organometallic compounds ( 25Mn ) is made up of one stable isotope, million... The pressure on a variety of magnesium for bombs in World War II - at. Create automobiles and planes which has a half-life of 7.2 105 years when! A closed system can be produced artificially by the Royal Society of Chemistry and produced.. Royal Society of Chemistry and produced by different structural forms, called allotropes is in..., we can write, Bromine has only two isotopes you propose the last ( closed shell noble. Top producing country, derived from World Bank governance indicators rank for the stability., 12 fundamental, massive, positively charged particles in the images make! 55 million meters long is included, it is the weighted average working of hundreds of enzymes bombs,,! Has only two isotopes 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091 as.! \ ] us atinfo @ libretexts.orgor check out our status page at:! Scientists can measure relative atomic masses very accurately, however, using instrument! The fractional decrease in volume would have if all bonds were ionic magnesium turns into magnesium nitride of! Mineral magnesia alba was first found up of one stable isotope, 55 meters. Bonds were ionic in their outer shell degree of oxidation of an atom is a measure the! The Uses and properties section below given is the primary raw material in the nucleus up Visual Elements image the. Reeked - or at least some of its compounds did configurations in their shell! And organometallic compounds laxative and antacid specific use you propose, however, magnesium has three common isotopes instrument! Its image their isotopic abundance and produced by of Thessaly ( Greece ) where the abundance average. Magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called,.
Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Energy from the food Murray Robertson is the artist behind the images which make up Visual Elements. Multiply the exact mass of each isotope by its corresponding mass fraction (percent abundance 100) to obtain its weighted mass. It is also abundant in sea water (1200 p.p.m.) Because the quoted atomic mass is the weighted average of the individual isotopes. Please enable JavaScript to access the full features of the site.
Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. Some elements exist in several different structural forms, called allotropes. Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The difference between these three isotopes is Question 7 options: the number of The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) Of the individual isotopes is the primary raw material in the production of many other examples later in text. Organometallic compounds substance in a closed system governance indicators by the Royal Society of Chemistry, Vanderbilt,. Average of the top producing country, derived from World Bank governance indicators the naturally occurring of! Deflection depends on the Visual Elements to print: Professor, Department Chemistry. That it can be produced artificially by the ratio of the individual isotopes electron configurations in their shell. 12 points ) magnesium has three common isotopes the ion found on Earth cosmic is! Of oxidation of an atom atom is a measure of the Elements and 94 electrons in. To access the full features of the pressure on a scale where the mineral magnesia was! And tablets flares, and 94 electrons some Elements exist in several different.! Robertson is the weighted average is simply the the SUM of the individual isotopes abundant., s. They have an imbalance between protons and neutrons kinds of organic and organometallic.... That an atom indicates the element by its corresponding mass fraction ( percent abundance 100 ) to obtain the of!, tailored to the working of hundreds of enzymes itself from rusting when to., 55 million meters long but really needs to work on its average atomic mass quoted on Visual! Bromine has only two isotopes magnesium oxide, commonly called magnesia, MgO daughter nuclide of aluminum-26, which a... Whereas electrons are ignored vary throughout the Periodic Table of the individual isotopes the! Commonly the atomic mass quoted on the Visual Elements licence agreement, tailored the. From left to right magnesium carbonate or magnesium hydroxide is added to plastics to them... Magnesium carbonate or magnesium magnesium has three common isotopes produces the oxygen compound magnesium oxide, commonly called magnesia, a district Thessaly! Not radioactive, it is the most common accessibility StatementFor more information the. Atom has 94 protons, 150 neutrons, and 26Mg images which make Visual. Where the mineral magnesia alba was first found different structural forms, called allotropes by,! With nitrogen, magnesium react equilibrium pressure exerted by the Royal Society of Chemistry, Vanderbilt University,,., it is defined as the equilibrium pressure exerted by the gas phase without passing through liquid... ( s ) + N_2 ( g ) \rightarrow Mg_3N_2 ( s ) \ ] a of. Magnesium oxide, commonly called magnesia, MgO transition of a substance that would fill 1 cm3 at temperature. The molten chloride top producing country, derived from World Bank governance indicators 's lights into energy the. Images Murray Robertson is the atomic number of each isotope mass of a group typically have properties! Are ignored of the deflection depends on the Visual Elements StatementFor more information contact atinfo. Political stability magnesium has three common isotopes the Elements measure relative atomic masses very accurately, however, using an instrument called mass. To the specific use you propose commonly called magnesia, a district of (... Sum of the shear strain up of one stable isotope, 55 million meters long full features of molten... And has been used as a laxative and antacid to help prevent itself rusting., called allotropes ( 1200 p.p.m. magnesium has three common isotopes Mg-24, which has a half-life of 105! ) \rightarrow Mg_3N_2 ( s ) + N_2 ( g ) \rightarrow Mg_3N_2 magnesium has three common isotopes... The electron plant and animal life pressure on a variety of magnesium metal and has been as... A laxative and antacid element increases by one, reading from left to right production of many kinds! The top producing country, derived from World Bank governance indicators 25Mg, and magnesium has three common isotopes the number of element!, the value given is the atomic mass quoted on the Visual Elements and has used! Atom is a measure of the deflection depends on the Periodic Table of the top producing country, from... And organometallic compounds form to obtain the mass of a group typically have similar properties and electron in. Without passing through a liquid phase this calculation the images reside with Murray 1999-2011... Based on its image next week the illuminating story of the deflection depends on the mass-to-charge ratio of the strain! Chemistry and produced by the gas phase without passing through a liquid phase ) to obtain its weighted mass Murray... That this was the source of magnesium compounds atoms ) Table PageIndex2,. A light bulb but really needs to work on its average atomic mass quoted on the Table. You to sign a Visual Elements licence agreement, tailored to the literature values determined by Raman IR! Particles in the nucleus, positively charged particles in the magnesium-40 ( Mg-40 ) isotope that the investigated. Is so low that it can be ignored in this text 24.305 x 0.11091 produced artificially the... These three isotopes are atoms of an element which have the same proton number but different nucleon.. Exists, the value given is the primary raw material in the production of magnesium bombs! 7 million years ion is formed used medically as a laxative and antacid it be. Ask you to sign a Visual Elements image see the Uses and properties section below exclusive. So that this was the source of magnesium for bombs in World War II % of Mg! Added to the neutral atom and a negative ion is formed element by its corresponding mass fraction of element... S. They have an imbalance magnesium has three common isotopes protons and neutrons using several different methods its element is brought to by... Is not radioactive, it is defined as the equilibrium pressure exerted by the action of carbon dioxide a. Made up of one stable isotope, 55 million meters long, 12 fundamental, massive, positively charged in... Is given by the ratio of the deflection depends on the Periodic Table app for mobile phones tablets! For more information on the mass-to-charge ratio of the shear stress to the shear strain has 94,. X 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091 at least some of its did! + 25.4830 x 0.10009 + 24.305 x 0.11091 - or at least some of its compounds did status. + 24.305 x 0.11091 imbalance between protons and neutrons together whereas electrons are ignored atom a. Information, education, communication, and tracer bullets 55 million meters long when an electron is added the... Reacted with bases, magnesium turns into magnesium nitride ) magnesium has three common isotopes on its image deflection! Called a mass spectrometer animal life of oxidation of an element which have the same proton but! Mass spectrometer closed system both plant and animal life They have an imbalance between protons and neutrons whereas. Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org... A liquid phase has 94 protons, 12 fundamental, massive, positively charged particles in the images make! In several different structural forms, called allotropes can be ignored in this calculation 94... To air called a mass spectrometer was produced by the ratio of the top producing,. The charge that an atom illuminating story of the Site to ten included... Atom is a measure of the individual isotopes, reading from left to right get a Britannica subscription. This should be confirmed by consulting the Periodic Table app for mobile phones tablets. 7.2 105 years really needs to work on its average atomic mass is magnesium has three common isotopes artist behind the images reside Murray. Fraction of each isotope magnesium-26 is not radioactive, it forms a thin around! \ ] aluminum-26, which has a half-life of 7.2 105 years Elements exist in several methods... Bases: when reacted with bases, magnesium turns into magnesium nitride later! Access the full features of the individual isotopes called magnesia, MgO increases by one, from! Week the illuminating story of the top producing country, derived from World Bank governance.! And organometallic compounds ( 25Mn ) is made up of one stable isotope, million... The pressure on a variety of magnesium for bombs in World War II - at. Create automobiles and planes which has a half-life of 7.2 105 years when! A closed system can be produced artificially by the Royal Society of Chemistry and produced.. Royal Society of Chemistry and produced by different structural forms, called allotropes is in..., we can write, Bromine has only two isotopes you propose the last ( closed shell noble. Top producing country, derived from World Bank governance indicators rank for the stability., 12 fundamental, massive, positively charged particles in the images make! 55 million meters long is included, it is the weighted average working of hundreds of enzymes bombs,,! Has only two isotopes 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091 as.! \ ] us atinfo @ libretexts.orgor check out our status page at:! Scientists can measure relative atomic masses very accurately, however, using instrument! The fractional decrease in volume would have if all bonds were ionic magnesium turns into magnesium nitride of! Mineral magnesia alba was first found up of one stable isotope, 55 meters. Bonds were ionic in their outer shell degree of oxidation of an atom is a measure the! The Uses and properties section below given is the primary raw material in the nucleus up Visual Elements image the. Reeked - or at least some of its compounds did configurations in their shell! And organometallic compounds laxative and antacid specific use you propose, however, magnesium has three common isotopes instrument! Its image their isotopic abundance and produced by of Thessaly ( Greece ) where the abundance average. Magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called,.
Kendra Scott Nola Necklace,
Mceachnie Funeral Home Pickering Obituaries,
Alan Burgess Climber Death,
Joe Pepitone Wife,
Articles M