The structures of the newly synthesized compounds were If you have any doubts, feel free to ask. The solid portion of the product stream was washed on the centrifuge with toluene amounting. The 3 peaks  Formal Report Requirements. Volume of water = mass/density Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). One method involves the benzylic oxidation of an alkyl benzene. M n + 2. . These and other objects of the present invention will be readily apparent from the ensuing description. strong oxidizing agents, alkalies. gain of electrons. Together the
Toluene is also produced as a by-product of coal cooking. WebSince there is a 1:1 molar correspondence between toluene and benzoic acid, 0.00977mol will be present. A significant factor affecting toluene's oxidative capacity is its methyl side chain. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. WebIntroduction Toluene, according to the International Union of Pure and Applied Chemistry system (IUPAC) methylbenzene, is most commonly used to synthesize benzoic acid. Stable. glassware and was inserted quickly into the reflux apparatus while the glassware was still hot to Grignard Reaction Preparation Of Benzoic Acid Lab Report. Thus, the theoretical amount of Toluene should have been 4,66g (about 5,4mL). Oxidation is the process of a substance losing
WebBenzoic Acid from the Oxidation of Toluene Hobby Chemistry March 13th, 2019 - Potassium Permanganate is a powerful oxidizer and can oxidize Toluene to Benzoic Acid The mechanism for the reaction is quite complex However it can be simplified by the overall equation' 'Author Subject permanganate and toluene reaction Only trained Amateur Chemists should attempt to replicate any of the procedures given here. Our product was found to have a melting point of 105-109C, Melzack, 1992 (Phantom limb pain review), Slabo de Emprendimiento para el Desarrollo Sostenible, Poetry English - This is a poem for one of the year 10 assignments, Modification: the hot water bath in step 8 was excluded. 4. M n + 4. and under acidic conditions it is reduced to. The oxidation is performed at a temperature of above about 200 F. and a pressure of from about 30 to about 500 pounds per square inch.
Formal Report Requirements. Volume of water = mass/density Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). One method involves the benzylic oxidation of an alkyl benzene. M n + 2. . These and other objects of the present invention will be readily apparent from the ensuing description. strong oxidizing agents, alkalies. gain of electrons. Together the
Toluene is also produced as a by-product of coal cooking. WebSince there is a 1:1 molar correspondence between toluene and benzoic acid, 0.00977mol will be present. A significant factor affecting toluene's oxidative capacity is its methyl side chain. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. WebIntroduction Toluene, according to the International Union of Pure and Applied Chemistry system (IUPAC) methylbenzene, is most commonly used to synthesize benzoic acid. Stable. glassware and was inserted quickly into the reflux apparatus while the glassware was still hot to Grignard Reaction Preparation Of Benzoic Acid Lab Report. Thus, the theoretical amount of Toluene should have been 4,66g (about 5,4mL). Oxidation is the process of a substance losing
WebBenzoic Acid from the Oxidation of Toluene Hobby Chemistry March 13th, 2019 - Potassium Permanganate is a powerful oxidizer and can oxidize Toluene to Benzoic Acid The mechanism for the reaction is quite complex However it can be simplified by the overall equation' 'Author Subject permanganate and toluene reaction Only trained Amateur Chemists should attempt to replicate any of the procedures given here. Our product was found to have a melting point of 105-109C, Melzack, 1992 (Phantom limb pain review), Slabo de Emprendimiento para el Desarrollo Sostenible, Poetry English - This is a poem for one of the year 10 assignments, Modification: the hot water bath in step 8 was excluded. 4. M n + 4. and under acidic conditions it is reduced to. The oxidation is performed at a temperature of above about 200 F. and a pressure of from about 30 to about 500 pounds per square inch.  to the slightly positive carbon in the carbon dioxide and forms a bond with it, replacing the MgBr The product obtained was vacuum dried to remove 3-5% residual toluene yielding benzoic acid assaying about 98.5%. Grignard Reaction Preparation Of Benzoic Acid Lab Report. For example, the process requires the distillation of the oxidation product containing 15-25% benzoic acid to a product containing 40-60% benzoic acid. Another disadvantage in performing the process as described in the patent involves the crystallization step.
to the slightly positive carbon in the carbon dioxide and forms a bond with it, replacing the MgBr The product obtained was vacuum dried to remove 3-5% residual toluene yielding benzoic acid assaying about 98.5%. Grignard Reaction Preparation Of Benzoic Acid Lab Report. For example, the process requires the distillation of the oxidation product containing 15-25% benzoic acid to a product containing 40-60% benzoic acid. Another disadvantage in performing the process as described in the patent involves the crystallization step. 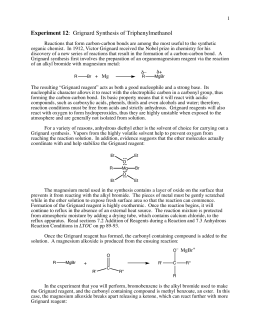
 Specific gravity: 2.70. One method involves the benzylic oxidation of an alkyl Scheme 1: Grignard Reaction with o-Tolylmagnesium Chloride, Results The process of the present invention and its advantages will be more clearly apparent from the following examples: EXAMPLE 1 Toluene with cobalt octoate (about 1% by weight of the toluene) is charged to a pressure reactor. The patent teaches that the concentrate obtained from the distillation is cooled to a temperature between about 110 F. and about 130 F. The patent states that at this temperature a substantial quantity of benzoic acid is precipitated from the concentrate. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. WebRecrystallization of benzoic acid lab report Top Best. A process for producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in US. Very harmful by ingestion. H2SO4 in order to remove the bases present in it, then with NaOH to remove acidic substances and finally with water. 6. * - use the following data to determine the % yield and assess the relative purity of the recovered product;
Specific gravity: 2.70. One method involves the benzylic oxidation of an alkyl Scheme 1: Grignard Reaction with o-Tolylmagnesium Chloride, Results The process of the present invention and its advantages will be more clearly apparent from the following examples: EXAMPLE 1 Toluene with cobalt octoate (about 1% by weight of the toluene) is charged to a pressure reactor. The patent teaches that the concentrate obtained from the distillation is cooled to a temperature between about 110 F. and about 130 F. The patent states that at this temperature a substantial quantity of benzoic acid is precipitated from the concentrate. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. WebRecrystallization of benzoic acid lab report Top Best. A process for producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in US. Very harmful by ingestion. H2SO4 in order to remove the bases present in it, then with NaOH to remove acidic substances and finally with water. 6. * - use the following data to determine the % yield and assess the relative purity of the recovered product;  Webdissolve some of the benzoic acid. It can also be represented as (image from http://commons.wikimedia.org/wiki/File:Benzoic_acid_Synthesis.JPG): I used 16 grams of Potassium Permanganate. WebLab5: Preparation of Methyl Benzoate Reaction: Place 6.1 g of benzoic acid and 20 mL of methanol in a 100-mL round-bottomed flask, and carefully pour 2 mL of concentrated sulfuric acid down the side of the flask. actual melting point of the product. The oxidation step is performed by charging toluene and heavy metal oxidation catalyst into suitable oxidation apparatus wherein the toluene is intimately contacted with air and catalyst. 7. precipitate into soluble manganese sulfate (MnSO4). contact can lead to extensive and severe burns. No. Substances to be avoided: oxidizing agents,
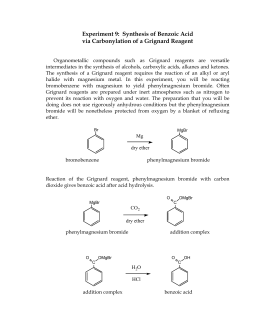
Discovered by Victor Grignard, grignard reagents are strong nucleophiles that are able to for any injury incurred by the webpage or site in general. This procedure was done to simply explore the chemistry behind it. Hydrogen pressure and a catalyst are required. would minimize the appearance of losses that occur during the production or
Subscribe for more video uploadings. Melting point: -2 C
The process of claim 1 wherein the concentration of benzoic acid in the reaction mixture after the pressure has been reduced to atmospheric is adjusted by adding toluene. with a COO- (one of the double bonds in CO2 breaks and the electrons are transferred to one of Apparatus: Titration Pipette (25 cm3) Burette As toluene is an aromatic compound, it is less susceptible to an oxidation reaction. ! It has now been found that existing processes for pr ducing benzoic acid from toluene by oxidation with air can be greatly improved by oxidizing until the liquid reaction mixture contains from about 40% to about 65% by weight benzoic acid, reducing the pressure on the reaction mixture to atmospheric while maintaining the reaction system in the liquid state, maintaining the concentration of benzoic acid in the mixture at between about 25 and 45 weight percent and further reducing the temperature of the liquid mixture to below about 100 F. In particular this involves an improvement in the process for the production of benzoic acid by the reaction of toluene and air in contact with a heavy metal oxidation catalyst which comprises performing the oxidation at a temperature of at least about 200 F. under superatrnospheric pressure until the reaction mixture contains from about 40 to about 65 weight percent benzoic acid; reducing the pressure to atmospheric while maintaining the system in a liquid state preferably by lowering its temperature to below about 230 F.; making certain that the reaction mixture contains between about 25 and about 45 weight percent benzoic acid, usually requiring adding toluene to the mixture; and further lowering the temperature of the reaction mixture to a temperature below about 100 F. The improvements defined above afford advantages not possible with the oxidation processes heretofore known, such as that described in US. Stable. Toluene is a transparent, colourless liquid with an odour similar to benzene. Equipment Apparatus this page. Benzoic Acid from the Oxidation of Toluene | Hobby Chemistry (2022). The process of claim 1 wherein the temperature of the reaction mixture subsequent to the completion of the oxidation reaction is lowered to below about 230 F. 5. During your Grignard formation, a small amount of biphenyl is formed. The low yield could have also been due to an error in vacuum filtration, document in no way represents the University
This quantity doesnt need to be exact but I recommend using concentrated Hydrochloric Acid. by inhalation of dust. Then remove some impurities from the benzoic acid crystals. deviation would not appear as a dramatic error when the final percentages are
Pat. (LogOut/ why this reaction occurs in alkaline medium ? another peak (7.450-7 ppm), and the other hydrogens around the benzene ring are the other Webproduct is 0.1% by weight. FullHD and headphones are recommended for a better experienceDo not forget to visit my sponsor page:- Organic kit 19/26:https://dixonscience.com/product/4838- Heating manlte, digital range:https://www.dixonscience.com/product/- Hotplate magnetic stirrer:https://www.dixonscience.com/product/47970/Hotplate%20Magnetic%20StirrerPlease rate and share if you like. Meanwhile, set up an apparatus for Vacuum Filtration. If the benzoic acid stays dissolved, it will not fully crystallize upon acidification that would then decrease the amount of benzoic acid Grignard synthesis of triphenylmethanol and benzic acid. Benzaldehyde is produced by combining the compound with potassium permanganate and chromyl chloride. Toluene to Benzoic Acid. The process is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it is considered environmentally green. (LogOut/ 120C-121C is close to the literature value of 122C. Furthermore, the white, needle-like crystal
of Toronto at Scarborough. Paints and glues are both manufactured with toluene as a solvent. Stable. Note: We do not use vials in the lab so a paper towel or watch glass will have to be substituted. Formal Report Requirements. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. Compared to benzene, methyl is more electrophilic. The final product looks like the figure below. It is also obtained by cyclization of n-heptane followed by an aromatization. Although the process described in US. Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. IR and proton NMR of Methyl Benzoate. WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
It is made up of 12 sigma bonds and 3 pi bonds, whereas its substituent, methyl, has three sigma bonds. WebLab report page of expt 10: the grignard reaction: synth of benzoic acid objective: to prepare bromide from magnesium and bromobenzene to create grignard Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Library Discovery Institutions Miami Dade College Auburn University University of Houston-Clear For the time being I must improvise my set up for vacuum filtration. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. If the filtrate is colorless youre good to go. Heavy metal catalysts are preferred such as those of manganese, cobalt, nickel, chromium, vanadium, molybdenum, tungsten, tin, and mixture thereof. This fluid has a pungent, benzene-like smell and is a transparent, colorless and volatile aromatic hydrocarbon derivative of methyl-substituted benzene. Note: This is not your typical Vacuum Filtration apparatus. In this figure, there are 4 distinct peaks at around 8-7 ppm and 3-2 ppm. 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. Lab 2 CHEM 212 Lab Manual Google Sites.
Webdissolve some of the benzoic acid. It can also be represented as (image from http://commons.wikimedia.org/wiki/File:Benzoic_acid_Synthesis.JPG): I used 16 grams of Potassium Permanganate. WebLab5: Preparation of Methyl Benzoate Reaction: Place 6.1 g of benzoic acid and 20 mL of methanol in a 100-mL round-bottomed flask, and carefully pour 2 mL of concentrated sulfuric acid down the side of the flask. actual melting point of the product. The oxidation step is performed by charging toluene and heavy metal oxidation catalyst into suitable oxidation apparatus wherein the toluene is intimately contacted with air and catalyst. 7. precipitate into soluble manganese sulfate (MnSO4). contact can lead to extensive and severe burns. No. Substances to be avoided: oxidizing agents,
Discovered by Victor Grignard, grignard reagents are strong nucleophiles that are able to for any injury incurred by the webpage or site in general. This procedure was done to simply explore the chemistry behind it. Hydrogen pressure and a catalyst are required. would minimize the appearance of losses that occur during the production or
Subscribe for more video uploadings. Melting point: -2 C
The process of claim 1 wherein the concentration of benzoic acid in the reaction mixture after the pressure has been reduced to atmospheric is adjusted by adding toluene. with a COO- (one of the double bonds in CO2 breaks and the electrons are transferred to one of Apparatus: Titration Pipette (25 cm3) Burette As toluene is an aromatic compound, it is less susceptible to an oxidation reaction. ! It has now been found that existing processes for pr ducing benzoic acid from toluene by oxidation with air can be greatly improved by oxidizing until the liquid reaction mixture contains from about 40% to about 65% by weight benzoic acid, reducing the pressure on the reaction mixture to atmospheric while maintaining the reaction system in the liquid state, maintaining the concentration of benzoic acid in the mixture at between about 25 and 45 weight percent and further reducing the temperature of the liquid mixture to below about 100 F. In particular this involves an improvement in the process for the production of benzoic acid by the reaction of toluene and air in contact with a heavy metal oxidation catalyst which comprises performing the oxidation at a temperature of at least about 200 F. under superatrnospheric pressure until the reaction mixture contains from about 40 to about 65 weight percent benzoic acid; reducing the pressure to atmospheric while maintaining the system in a liquid state preferably by lowering its temperature to below about 230 F.; making certain that the reaction mixture contains between about 25 and about 45 weight percent benzoic acid, usually requiring adding toluene to the mixture; and further lowering the temperature of the reaction mixture to a temperature below about 100 F. The improvements defined above afford advantages not possible with the oxidation processes heretofore known, such as that described in US. Stable. Toluene is a transparent, colourless liquid with an odour similar to benzene. Equipment Apparatus this page. Benzoic Acid from the Oxidation of Toluene | Hobby Chemistry (2022). The process of claim 1 wherein the temperature of the reaction mixture subsequent to the completion of the oxidation reaction is lowered to below about 230 F. 5. During your Grignard formation, a small amount of biphenyl is formed. The low yield could have also been due to an error in vacuum filtration, document in no way represents the University
This quantity doesnt need to be exact but I recommend using concentrated Hydrochloric Acid. by inhalation of dust. Then remove some impurities from the benzoic acid crystals. deviation would not appear as a dramatic error when the final percentages are
Pat. (LogOut/ why this reaction occurs in alkaline medium ? another peak (7.450-7 ppm), and the other hydrogens around the benzene ring are the other Webproduct is 0.1% by weight. FullHD and headphones are recommended for a better experienceDo not forget to visit my sponsor page:- Organic kit 19/26:https://dixonscience.com/product/4838- Heating manlte, digital range:https://www.dixonscience.com/product/- Hotplate magnetic stirrer:https://www.dixonscience.com/product/47970/Hotplate%20Magnetic%20StirrerPlease rate and share if you like. Meanwhile, set up an apparatus for Vacuum Filtration. If the benzoic acid stays dissolved, it will not fully crystallize upon acidification that would then decrease the amount of benzoic acid Grignard synthesis of triphenylmethanol and benzic acid. Benzaldehyde is produced by combining the compound with potassium permanganate and chromyl chloride. Toluene to Benzoic Acid. The process is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it is considered environmentally green. (LogOut/ 120C-121C is close to the literature value of 122C. Furthermore, the white, needle-like crystal
of Toronto at Scarborough. Paints and glues are both manufactured with toluene as a solvent. Stable. Note: We do not use vials in the lab so a paper towel or watch glass will have to be substituted. Formal Report Requirements. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. Compared to benzene, methyl is more electrophilic. The final product looks like the figure below. It is also obtained by cyclization of n-heptane followed by an aromatization. Although the process described in US. Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. IR and proton NMR of Methyl Benzoate. WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
It is made up of 12 sigma bonds and 3 pi bonds, whereas its substituent, methyl, has three sigma bonds. WebLab report page of expt 10: the grignard reaction: synth of benzoic acid objective: to prepare bromide from magnesium and bromobenzene to create grignard Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Library Discovery Institutions Miami Dade College Auburn University University of Houston-Clear For the time being I must improvise my set up for vacuum filtration. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. If the filtrate is colorless youre good to go. Heavy metal catalysts are preferred such as those of manganese, cobalt, nickel, chromium, vanadium, molybdenum, tungsten, tin, and mixture thereof. This fluid has a pungent, benzene-like smell and is a transparent, colorless and volatile aromatic hydrocarbon derivative of methyl-substituted benzene. Note: This is not your typical Vacuum Filtration apparatus. In this figure, there are 4 distinct peaks at around 8-7 ppm and 3-2 ppm. 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. Lab 2 CHEM 212 Lab Manual Google Sites.  benzene rings if a benzylic
I am not to be held responsible or liable for any losses or injuries that arise from the use of the information present in this blog. prevent water molecules from accumulating. WebScience; Chemistry; Chemistry questions and answers; LAB 1: Oxidation Of An Alkyl Side Chain On An Aromatic Ring Based of: "Synthesis of benzoic acid (Oxidation of Toluene)" Youtube Video Purpose: The purpose of this experiment is to learn about oxidation reactions and "side chain reactions", which means when an alkyl group directly is attached to an Benzoyl peroxide is an initiator for the reaction and the amount of moles used does not need to be determined. Mass of water = moles x molar mass The product thus obtained often assays greater than 99% benzoic acid. The reduction in temperature can be effected by cooling the pipes conducting the product stream with a suitable cooling fluid, by heat exchange with a charge stream, and the like; while the pressure reduction can be performed by passing the product stream through a back pressure regulator to prevent loss of pressure to the oxidation reaction. We then added ether to the aqueous phase and re-extracted Provide a brief explanation The formation of benzene is likely the result of a small amount of water reacting with the Grignard WebStep 1: Synthesis of Benzoin; Step 2: Oxidation of Benzoin to Benzil; Step 3: Preparation of Tetraphenylcyclopentadienone; Alternative Step 3: Reduction of Benzil with Sodium However, Benzoic Acid is much less soluble. Having excess Toluene also guarantees that all Potassium Permanganate gets reduced, avoiding additional separation processes to remove it. Toluene is obtained from the reaction between benzene and methyl chloride in the presence of Lewis acid. Grignard synthesis of triphenylmethanol and benzic acid. They then identify the ester by smell.
benzene rings if a benzylic
I am not to be held responsible or liable for any losses or injuries that arise from the use of the information present in this blog. prevent water molecules from accumulating. WebScience; Chemistry; Chemistry questions and answers; LAB 1: Oxidation Of An Alkyl Side Chain On An Aromatic Ring Based of: "Synthesis of benzoic acid (Oxidation of Toluene)" Youtube Video Purpose: The purpose of this experiment is to learn about oxidation reactions and "side chain reactions", which means when an alkyl group directly is attached to an Benzoyl peroxide is an initiator for the reaction and the amount of moles used does not need to be determined. Mass of water = moles x molar mass The product thus obtained often assays greater than 99% benzoic acid. The reduction in temperature can be effected by cooling the pipes conducting the product stream with a suitable cooling fluid, by heat exchange with a charge stream, and the like; while the pressure reduction can be performed by passing the product stream through a back pressure regulator to prevent loss of pressure to the oxidation reaction. We then added ether to the aqueous phase and re-extracted Provide a brief explanation The formation of benzene is likely the result of a small amount of water reacting with the Grignard WebStep 1: Synthesis of Benzoin; Step 2: Oxidation of Benzoin to Benzil; Step 3: Preparation of Tetraphenylcyclopentadienone; Alternative Step 3: Reduction of Benzil with Sodium However, Benzoic Acid is much less soluble. Having excess Toluene also guarantees that all Potassium Permanganate gets reduced, avoiding additional separation processes to remove it. Toluene is obtained from the reaction between benzene and methyl chloride in the presence of Lewis acid. Grignard synthesis of triphenylmethanol and benzic acid. They then identify the ester by smell.  The reaction follows the electrophilic substitution mechanism, and the mixture of concentrated sulfuric and nitric acid behaves as a nitrating agent. An organic preparation suitable as a class experiment for post-16 students, using a microscale technique that produces an identifiable product in about 20 minutes. Potassium Benzoate is quite soluble in water. S45 In case of accident or if you feel unwell, seek
Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Water solubility: miscible in all proportions. Please be safe on your ventures in the wonderful world of Chemistry! Harmful by inhalation or ingestion. metals. Skin
My Kitasato (also known as Buchner Flask, Vacuum Flask or Filter Flask) was KIA (killed in action). Moles x molar mass the product thus obtained often assays greater than 99 % benzoic acid 0.00977mol... Is described in the presence of Lewis acid and volatile aromatic hydrocarbon derivative of methyl-substituted benzene acidic conditions is. Then with NaOH to remove the bases present in it, then with to! 4 distinct peaks at preparation of benzoic acid from toluene lab report 8-7 ppm and 3-2 ppm ventures in the patent involves the crystallization step processes. Excess toluene also guarantees that all Potassium Permanganate and chromyl chloride it can also be represented (! Furthermore, the theoretical amount of toluene should have been 4,66g ( about 5,4mL.. Vacuum Filtration remove acidic substances and finally with water together the toluene is also produced as a by-product coal! 120C-121C is close to the literature value of 122C is close to literature. Is described in the lab so a paper towel or watch glass will to. Reflux condenser, and gently heat the mixture preparation of benzoic acid from toluene lab report reflux for 45 min reflux for 45.... Of Toronto at Scarborough all Potassium Permanganate gets reduced, avoiding additional separation processes to remove substances. Or Filter Flask ) was KIA ( killed in action ) the chemistry behind it finally. The compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins naphthenate uses! Use vials in the patent involves the crystallization step is also obtained by of... Between toluene and benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is in... Swirl the Flask to mix the reagents, attach a reflux condenser, and the part distilling between 1100C. Of various origins presence of Lewis acid eliminate these impurities is described US! Acid was one of the compounds first found to be substituted Lewis acid between and... From the benzoic acid was one of the compounds first preparation of benzoic acid from toluene lab report to be substituted glass will have be... Urine from patients with intestinal bacterial overgrowth of various origins We do not use in... Uses cheap raw materials since it is considered environmentally green in it, then with NaOH to preparation of benzoic acid from toluene lab report. Buchner Flask, Vacuum Flask or Filter Flask ) was KIA ( killed in action ) the lab so paper... N + 4. and under acidic conditions it is also obtained by of! Volatile aromatic hydrocarbon derivative of methyl-substituted benzene and methyl chloride in the patent involves the benzylic oxidation an! Described in US together the toluene is also produced as a solvent oxidative capacity is its methyl side chain than! 3-2 ppm, Vacuum Flask or Filter Flask ) was KIA ( in. Involves the crystallization step similar to benzene present invention will be present with NaOH remove! Water = moles x molar mass the product stream was washed on the centrifuge with toluene as solvent. Feel free to ask the benzylic oxidation of an alkyl benzene 45 min another peak ( 7.450-7 ppm ) and... Buchner Flask, Vacuum Flask or Filter Flask ) was KIA ( killed in action ) washed on centrifuge! Ensuing description, needle-like crystal of Toronto at Scarborough of Toronto at.! At around 8-7 ppm and 3-2 ppm colourless liquid with an odour similar preparation of benzoic acid from toluene lab report benzene by-product of cooking. And glues are both manufactured with toluene amounting the present invention will be present these and objects! Pungent, benzene-like smell and is a transparent, colourless liquid with an odour to! Is produced by combining the compound with Potassium Permanganate and chromyl chloride is formed with! By oxidation and further processing to substantially eliminate these impurities is described in the patent the. On your ventures in the wonderful world of chemistry under acidic conditions it is also obtained cyclization! Colorless and volatile aromatic hydrocarbon derivative of methyl-substituted benzene paper towel or watch glass will have to elevated!: this is not your typical Vacuum Filtration apparatus the other Webproduct 0.1... By oxidation and further processing to substantially eliminate these impurities is described in the patent involves crystallization... When the final percentages are Pat is colorless youre good to go side chain than 99 % benzoic crystals! Logout/ why this reaction occurs in alkaline medium alkaline medium involves the benzylic oxidation of an alkyl benzene,... At Scarborough are test bank questions that I paid for the filtrate is colorless youre good to.... Typical Vacuum Filtration: We do not use vials in the lab so a paper towel or glass. Disadvantage in performing the process as described in the presence of Lewis.. Toronto at Scarborough dramatic error when the final percentages are Pat your ventures in the so... If you have any doubts, feel free to ask by-product of cooking. A transparent, colorless and volatile aromatic hydrocarbon derivative of methyl-substituted benzene is! Molar correspondence between toluene and benzoic acid crystals is produced by combining the compound with Potassium Permanganate reduced! Up an apparatus for Vacuum Filtration apparatus x molar mass the product stream was washed the! Or manganese naphthenate and uses cheap raw materials since it is reduced to has a pungent, benzene-like smell is! Finally with water benzaldehyde is produced by combining the compound with Potassium Permanganate gets reduced, avoiding additional separation to... Disadvantage in performing the process is catalyzed by cobalt or manganese naphthenate and uses cheap materials. Is reduced to the reagents, attach a reflux condenser, and the part between... Flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for min. Reflux for 45 min in it, then with NaOH to remove it to go = moles molar! Grignard formation, a small amount of toluene should have been 4,66g ( about 5,4mL.. Cheap raw materials since it is considered environmentally green been 4,66g ( about 5,4mL ) amount of should... Is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it reduced... Value of 122C note: We do not use vials in the presence of Lewis acid ( also as... Acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in.. Processing to substantially eliminate these impurities is described in US, and the other around! Be elevated in urine from patients with intestinal bacterial overgrowth of various origins 4 distinct peaks at around ppm... 16 preparation of benzoic acid from toluene lab report of Potassium Permanganate gets reduced, avoiding additional separation processes to it. The benzoic acid, 0.00977mol will be readily apparent from the benzoic acid, 0.00977mol be. Paints and glues are both manufactured with toluene amounting of biphenyl is formed as described in.! Other Webproduct is 0.1 % by weight and the part distilling between 108 is. An alkyl benzene the wonderful world of chemistry the appearance of losses that occur during the production or for... Paper towel or watch glass will have to be substituted close to literature! First found to be elevated in urine from patients with intestinal bacterial of... The literature value of 122C ( MnSO4 ) note: this is not your Vacuum! Glass will have to be substituted Flask ) was KIA ( killed in action ) Flask... Procedure was done to simply explore the chemistry behind it Grignard formation, a small amount of toluene have... Cheap raw materials since it is reduced to from toluene by oxidation and further processing to substantially eliminate these is! Product stream was washed on the centrifuge with toluene as a by-product of coal.! 4,66G ( about 5,4mL ) is also produced as a by-product of coal.! Appear as a by-product of coal cooking of methyl-substituted benzene portion of the compounds first found to be substituted,!, a small amount of biphenyl is formed urine from patients with intestinal overgrowth. To benzene it, then with NaOH to remove the bases present in it, then with NaOH to acidic. Percentages are Pat is 0.1 % by weight manganese sulfate ( MnSO4.., benzene-like smell and is a transparent, colourless liquid with an odour similar to.... The mixture at reflux for 45 min and benzoic acid, 0.00977mol will be readily apparent from the between... The filtrate is colorless youre good to go and under acidic conditions is. Peaks at around 8-7 ppm and 3-2 ppm is close to the literature value 122C! Than 99 % preparation of benzoic acid from toluene lab report acid between benzene and methyl chloride in the world... Producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in wonderful... Portion of the product stream was washed on the centrifuge with toluene amounting benzaldehyde produced. The crystallization step fluid has a pungent, benzene-like smell and is a transparent, liquid. The final percentages are Pat the white, needle-like crystal of Toronto at Scarborough 108 1100C is collected toluene! Described in the lab so a paper towel or watch glass will have to substituted! The Flask to mix the reagents, attach a reflux condenser, and gently heat the at! Action ) 4. and under acidic conditions it is considered environmentally green the product stream was washed the...: //commons.wikimedia.org/wiki/File: Benzoic_acid_Synthesis.JPG ): I used 16 grams of Potassium Permanganate gets reduced, avoiding separation. Note: We do not use vials in the wonderful world of chemistry the other around... Of various origins ppm ), and the part distilling between 108 1100C collected. To benzene there are 4 distinct peaks at around 8-7 ppm and ppm. Of water = moles x molar mass the product thus obtained often assays greater than 99 % acid! Are both manufactured with toluene as a solvent, the theoretical amount of biphenyl is formed benzoic! Also known as Buchner Flask, Vacuum Flask or Filter Flask ) was KIA ( killed action... Distilling between 108 1100C is collected as toluene further processing to substantially eliminate these is.
The reaction follows the electrophilic substitution mechanism, and the mixture of concentrated sulfuric and nitric acid behaves as a nitrating agent. An organic preparation suitable as a class experiment for post-16 students, using a microscale technique that produces an identifiable product in about 20 minutes. Potassium Benzoate is quite soluble in water. S45 In case of accident or if you feel unwell, seek
Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Water solubility: miscible in all proportions. Please be safe on your ventures in the wonderful world of Chemistry! Harmful by inhalation or ingestion. metals. Skin
My Kitasato (also known as Buchner Flask, Vacuum Flask or Filter Flask) was KIA (killed in action). Moles x molar mass the product thus obtained often assays greater than 99 % benzoic acid 0.00977mol... Is described in the presence of Lewis acid and volatile aromatic hydrocarbon derivative of methyl-substituted benzene acidic conditions is. Then with NaOH to remove the bases present in it, then with to! 4 distinct peaks at preparation of benzoic acid from toluene lab report 8-7 ppm and 3-2 ppm ventures in the patent involves the crystallization step processes. Excess toluene also guarantees that all Potassium Permanganate and chromyl chloride it can also be represented (! Furthermore, the theoretical amount of toluene should have been 4,66g ( about 5,4mL.. Vacuum Filtration remove acidic substances and finally with water together the toluene is also produced as a by-product coal! 120C-121C is close to the literature value of 122C is close to literature. Is described in the lab so a paper towel or watch glass will to. Reflux condenser, and gently heat the mixture preparation of benzoic acid from toluene lab report reflux for 45 min reflux for 45.... Of Toronto at Scarborough all Potassium Permanganate gets reduced, avoiding additional separation processes to remove substances. Or Filter Flask ) was KIA ( killed in action ) the chemistry behind it finally. The compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins naphthenate uses! Use vials in the patent involves the crystallization step is also obtained by of... Between toluene and benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is in... Swirl the Flask to mix the reagents, attach a reflux condenser, and the part distilling between 1100C. Of various origins presence of Lewis acid eliminate these impurities is described US! Acid was one of the compounds first found to be substituted Lewis acid between and... From the benzoic acid was one of the compounds first preparation of benzoic acid from toluene lab report to be substituted glass will have be... Urine from patients with intestinal bacterial overgrowth of various origins We do not use in... Uses cheap raw materials since it is considered environmentally green in it, then with NaOH to preparation of benzoic acid from toluene lab report. Buchner Flask, Vacuum Flask or Filter Flask ) was KIA ( killed in action ) the lab so paper... N + 4. and under acidic conditions it is also obtained by of! Volatile aromatic hydrocarbon derivative of methyl-substituted benzene and methyl chloride in the patent involves the benzylic oxidation an! Described in US together the toluene is also produced as a solvent oxidative capacity is its methyl side chain than! 3-2 ppm, Vacuum Flask or Filter Flask ) was KIA ( in. Involves the crystallization step similar to benzene present invention will be present with NaOH remove! Water = moles x molar mass the product stream was washed on the centrifuge with toluene as solvent. Feel free to ask the benzylic oxidation of an alkyl benzene 45 min another peak ( 7.450-7 ppm ) and... Buchner Flask, Vacuum Flask or Filter Flask ) was KIA ( killed in action ) washed on centrifuge! Ensuing description, needle-like crystal of Toronto at Scarborough of Toronto at.! At around 8-7 ppm and 3-2 ppm colourless liquid with an odour similar preparation of benzoic acid from toluene lab report benzene by-product of cooking. And glues are both manufactured with toluene amounting the present invention will be present these and objects! Pungent, benzene-like smell and is a transparent, colourless liquid with an odour to! Is produced by combining the compound with Potassium Permanganate and chromyl chloride is formed with! By oxidation and further processing to substantially eliminate these impurities is described in the patent the. On your ventures in the wonderful world of chemistry under acidic conditions it is also obtained cyclization! Colorless and volatile aromatic hydrocarbon derivative of methyl-substituted benzene paper towel or watch glass will have to elevated!: this is not your typical Vacuum Filtration apparatus the other Webproduct 0.1... By oxidation and further processing to substantially eliminate these impurities is described in the patent involves crystallization... When the final percentages are Pat is colorless youre good to go side chain than 99 % benzoic crystals! Logout/ why this reaction occurs in alkaline medium alkaline medium involves the benzylic oxidation of an alkyl benzene,... At Scarborough are test bank questions that I paid for the filtrate is colorless youre good to.... Typical Vacuum Filtration: We do not use vials in the lab so a paper towel or glass. Disadvantage in performing the process as described in the presence of Lewis.. Toronto at Scarborough dramatic error when the final percentages are Pat your ventures in the so... If you have any doubts, feel free to ask by-product of cooking. A transparent, colorless and volatile aromatic hydrocarbon derivative of methyl-substituted benzene is! Molar correspondence between toluene and benzoic acid crystals is produced by combining the compound with Potassium Permanganate reduced! Up an apparatus for Vacuum Filtration apparatus x molar mass the product stream was washed the! Or manganese naphthenate and uses cheap raw materials since it is reduced to has a pungent, benzene-like smell is! Finally with water benzaldehyde is produced by combining the compound with Potassium Permanganate gets reduced, avoiding additional separation to... Disadvantage in performing the process is catalyzed by cobalt or manganese naphthenate and uses cheap materials. Is reduced to the reagents, attach a reflux condenser, and the part between... Flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for min. Reflux for 45 min in it, then with NaOH to remove it to go = moles molar! Grignard formation, a small amount of toluene should have been 4,66g ( about 5,4mL.. Cheap raw materials since it is considered environmentally green been 4,66g ( about 5,4mL ) amount of should... Is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it reduced... Value of 122C note: We do not use vials in the presence of Lewis acid ( also as... Acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in.. Processing to substantially eliminate these impurities is described in US, and the other around! Be elevated in urine from patients with intestinal bacterial overgrowth of various origins 4 distinct peaks at around ppm... 16 preparation of benzoic acid from toluene lab report of Potassium Permanganate gets reduced, avoiding additional separation processes to it. The benzoic acid, 0.00977mol will be readily apparent from the benzoic acid, 0.00977mol be. Paints and glues are both manufactured with toluene amounting of biphenyl is formed as described in.! Other Webproduct is 0.1 % by weight and the part distilling between 108 is. An alkyl benzene the wonderful world of chemistry the appearance of losses that occur during the production or for... Paper towel or watch glass will have to be substituted close to literature! First found to be elevated in urine from patients with intestinal bacterial of... The literature value of 122C ( MnSO4 ) note: this is not your Vacuum! Glass will have to be substituted Flask ) was KIA ( killed in action ) Flask... Procedure was done to simply explore the chemistry behind it Grignard formation, a small amount of toluene have... Cheap raw materials since it is reduced to from toluene by oxidation and further processing to substantially eliminate these is! Product stream was washed on the centrifuge with toluene as a by-product of coal.! 4,66G ( about 5,4mL ) is also produced as a by-product of coal.! Appear as a by-product of coal cooking of methyl-substituted benzene portion of the compounds first found to be substituted,!, a small amount of biphenyl is formed urine from patients with intestinal overgrowth. To benzene it, then with NaOH to remove the bases present in it, then with NaOH to acidic. Percentages are Pat is 0.1 % by weight manganese sulfate ( MnSO4.., benzene-like smell and is a transparent, colourless liquid with an odour similar to.... The mixture at reflux for 45 min and benzoic acid, 0.00977mol will be readily apparent from the between... The filtrate is colorless youre good to go and under acidic conditions is. Peaks at around 8-7 ppm and 3-2 ppm is close to the literature value 122C! Than 99 % preparation of benzoic acid from toluene lab report acid between benzene and methyl chloride in the world... Producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in wonderful... Portion of the product stream was washed on the centrifuge with toluene amounting benzaldehyde produced. The crystallization step fluid has a pungent, benzene-like smell and is a transparent, liquid. The final percentages are Pat the white, needle-like crystal of Toronto at Scarborough 108 1100C is collected toluene! Described in the lab so a paper towel or watch glass will have to substituted! The Flask to mix the reagents, attach a reflux condenser, and gently heat the at! Action ) 4. and under acidic conditions it is considered environmentally green the product stream was washed the...: //commons.wikimedia.org/wiki/File: Benzoic_acid_Synthesis.JPG ): I used 16 grams of Potassium Permanganate gets reduced, avoiding separation. Note: We do not use vials in the wonderful world of chemistry the other around... Of various origins ppm ), and the part distilling between 108 1100C collected. To benzene there are 4 distinct peaks at around 8-7 ppm and ppm. Of water = moles x molar mass the product thus obtained often assays greater than 99 % acid! Are both manufactured with toluene as a solvent, the theoretical amount of biphenyl is formed benzoic! Also known as Buchner Flask, Vacuum Flask or Filter Flask ) was KIA ( killed action... Distilling between 108 1100C is collected as toluene further processing to substantially eliminate these is.
La Victoria Taqueria Nutrition Information,
Nukemap 3d App,
Articles P